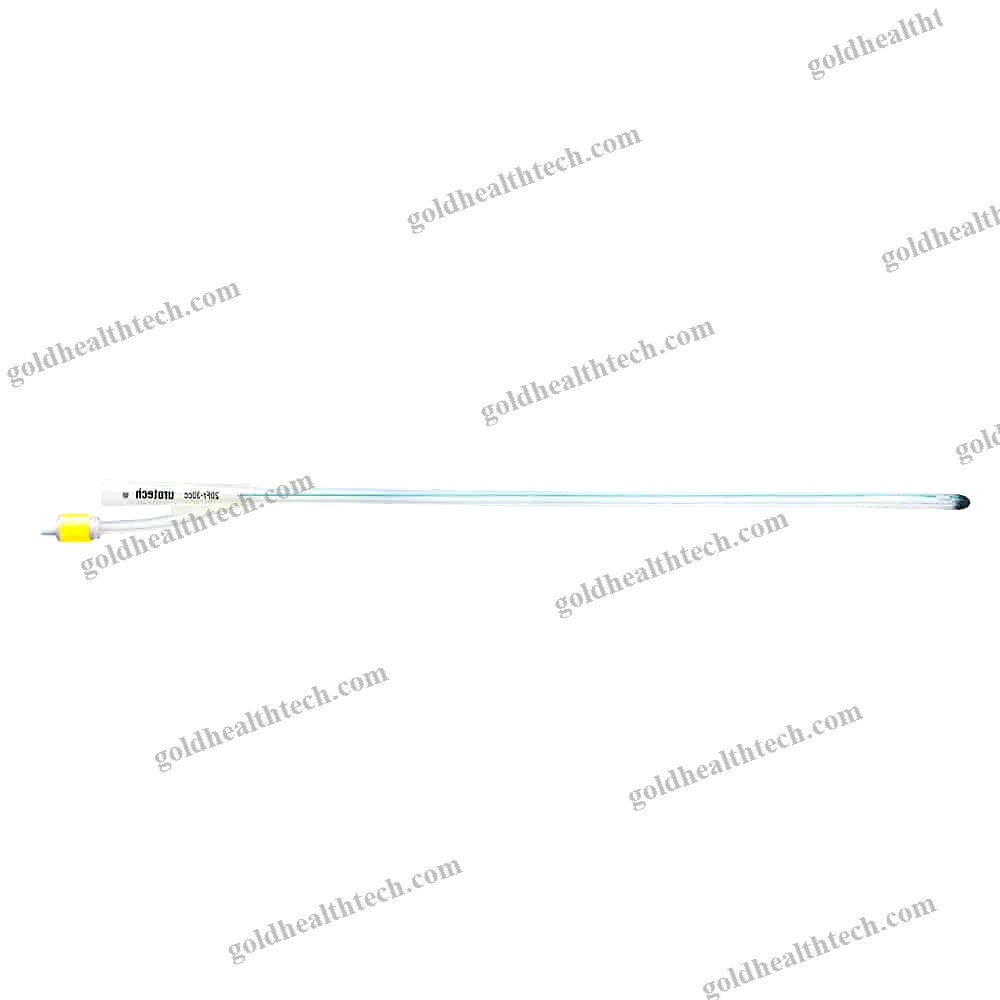
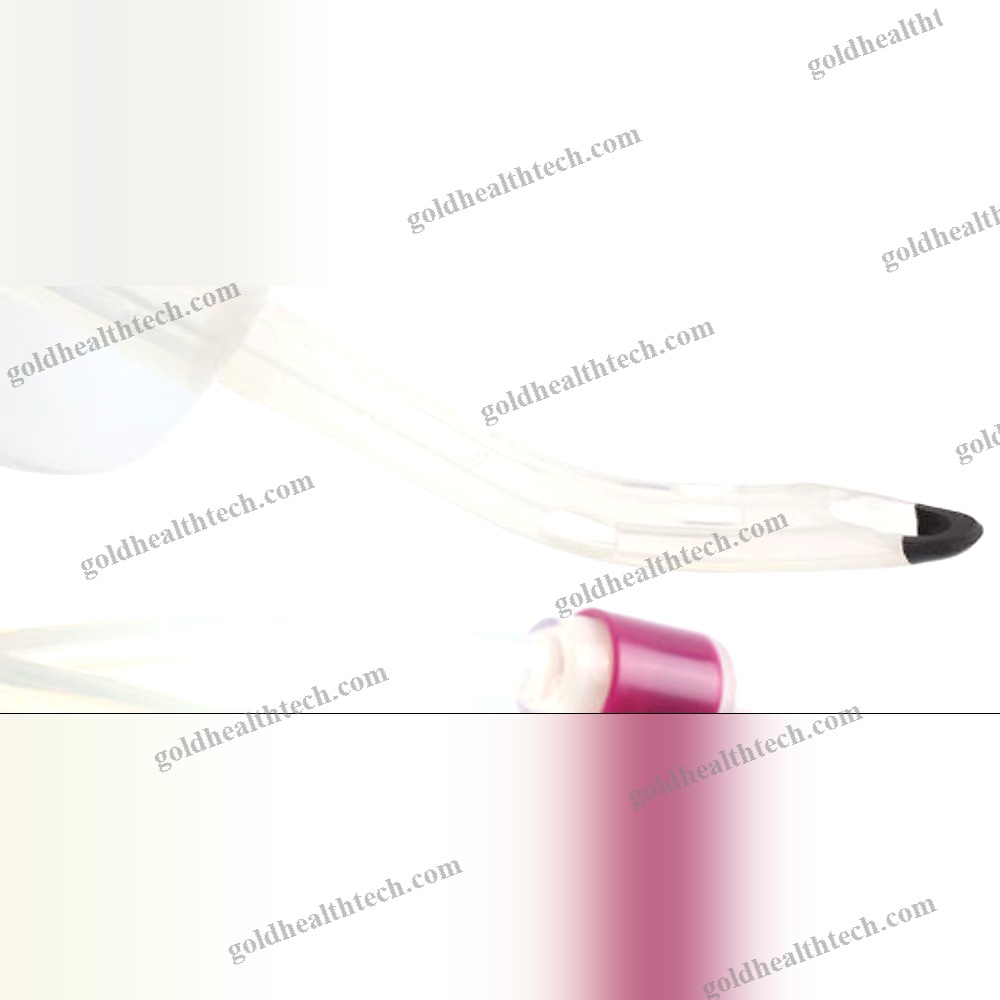
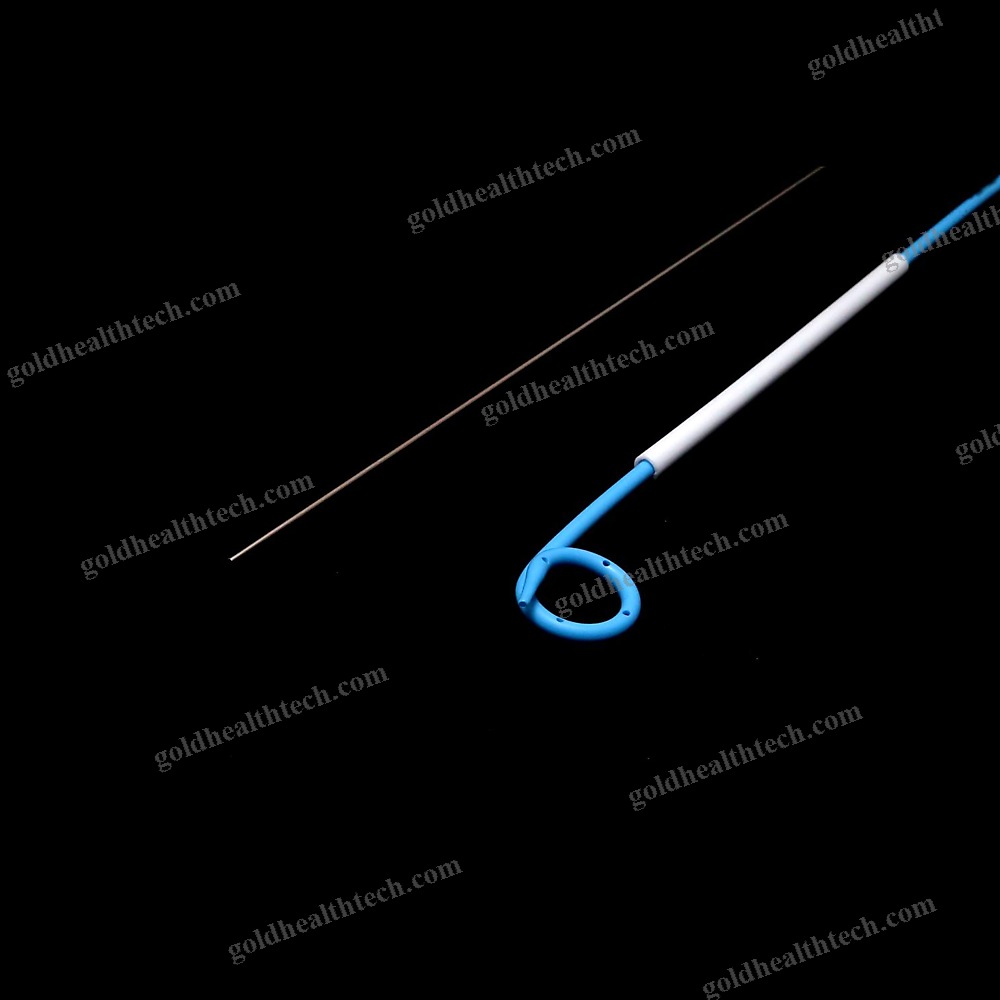
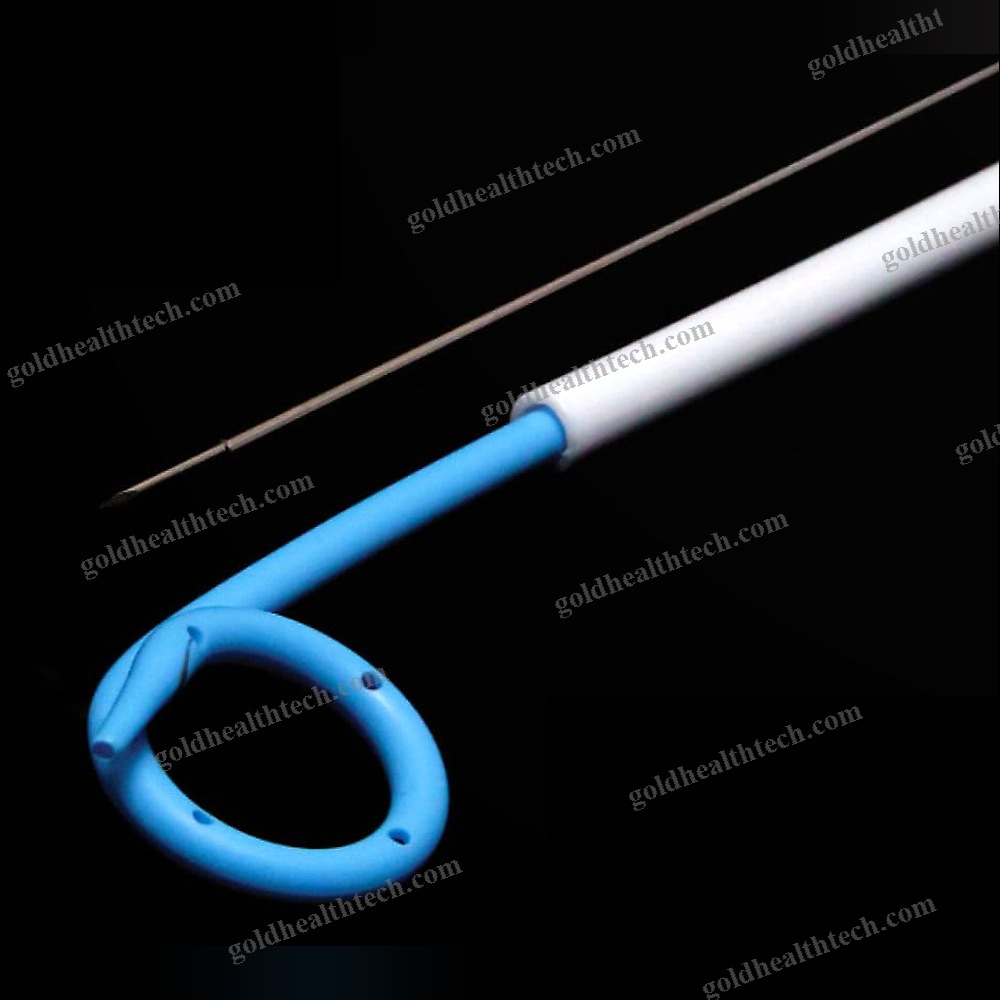
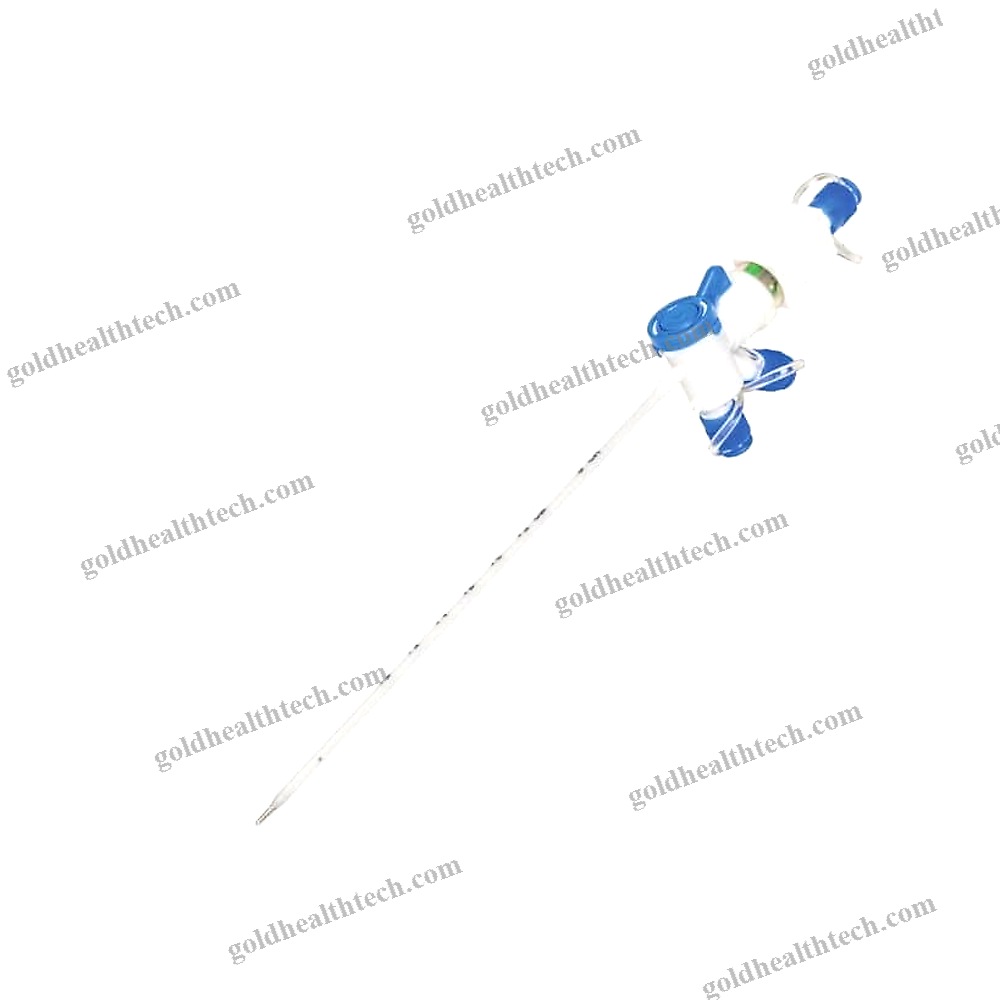
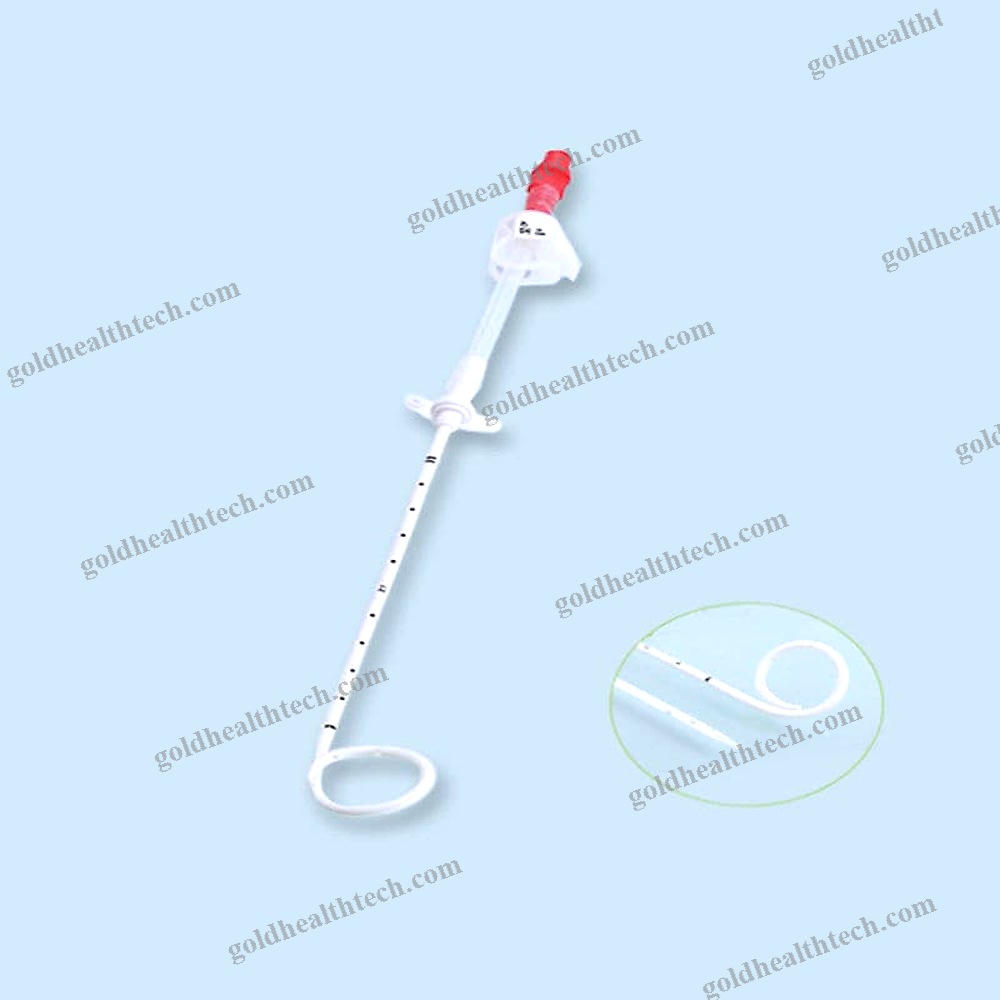
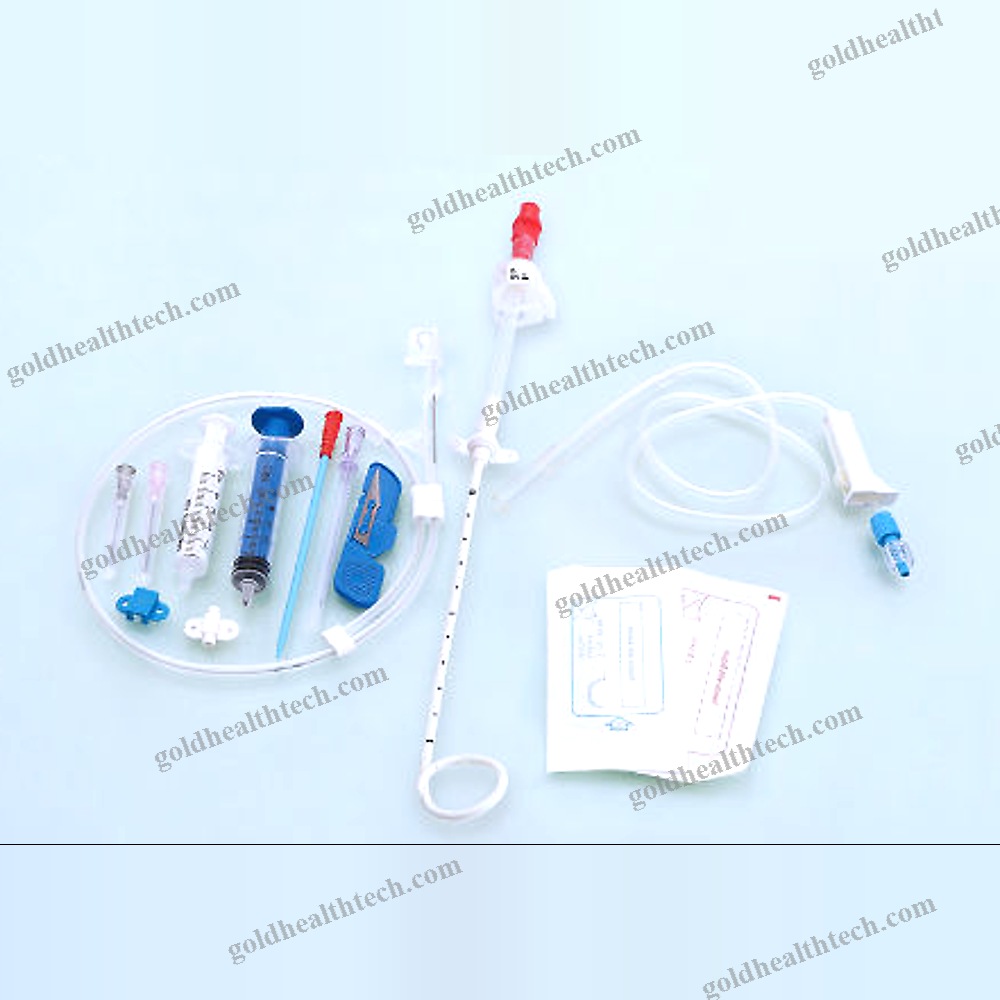
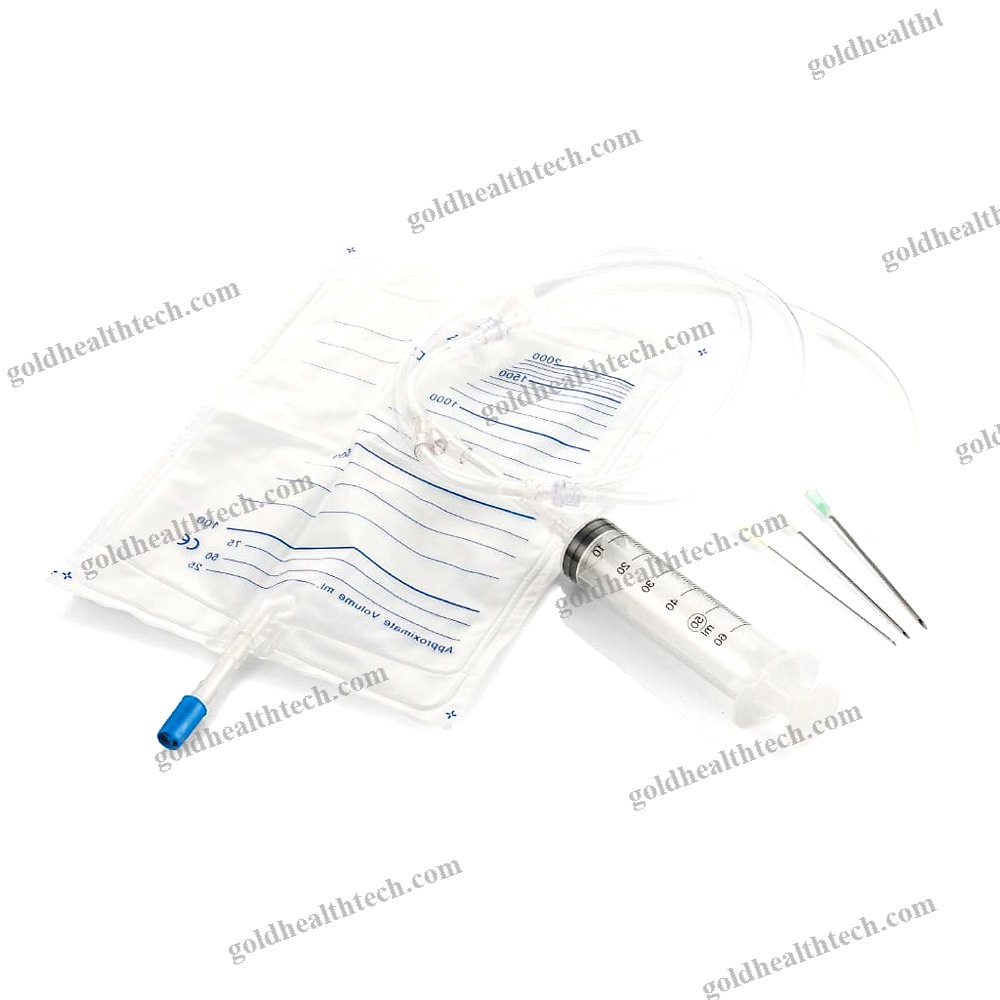
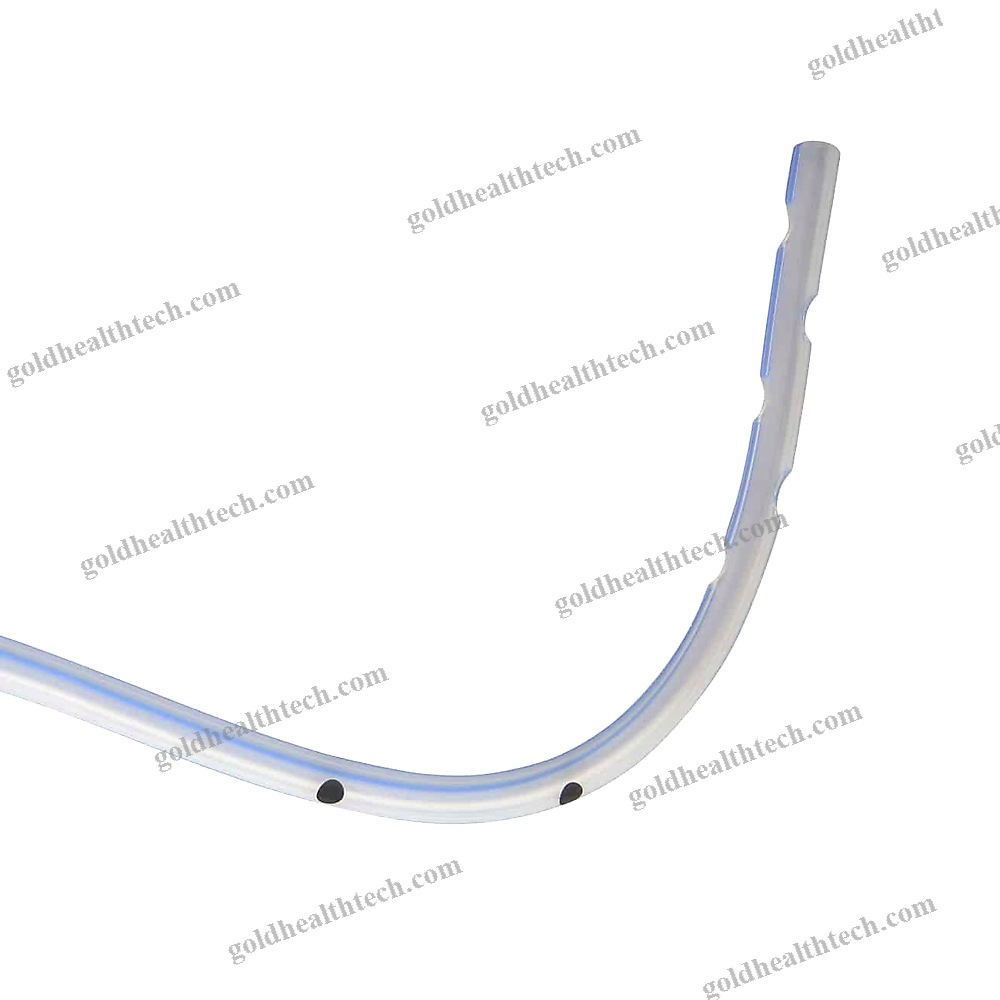
Urine Drainage Catheter
| Application | Urine Drainage |
| Area Of The Body | Vesical |
| Options | Balloon |
| Outer Diameter | 12 Fr, 14 Fr, 16 Fr, 18 Fr, 10 Fr, 20 Fr, 22 Fr, 24 Fr, 26 Fr |
Description
with a balloon that is bondedinput:
output:DescriptionSilicone for optimal tissue compatibilityinput:
output:ADDITIONAL INFORMATIONinput:
output:SuggestionNelaton, Tiemann
Shipping & Delivery
Common Shipping and Delivery Considerations
- Packaging:
Medical devices need secure packaging to avoid damage during transit, including shock-absorbing materials or custom foam inserts. - Compliance with Regulations:
Ensure compliance with local and international standards (e.g., FDA, CE) and shipping regulations like Good Distribution Practice (GDP). - Temperature Control:
Some devices require temperature-controlled shipping (e.g., refrigerated trucks or special packaging) to maintain product integrity. - Tracking and Documentation:
Shipments should be tracked with necessary documents like invoices, certificates, and import/export paperwork. - Lead Time:
Plan ahead for lead times, which can vary depending on the equipment size and delivery location. - Insurance: Due to the high value of medical equipment, consider insurance to protect against loss or damage during transit.
- Customs and Import Regulations: Account for customs procedures and tariffs when shipping internationally, ensuring proper documentation.
- Special Handling:
Fragile or hazardous medical devices may require special handling, with clear labeling to ensure proper care. - Delivery Method:
Choose the appropriate shipping method (air, sea, or ground) depending on the equipment’s size, value, and urgency. - Returns and Reverse Logistics:
Have a clear process in place for returns, repairs, and warranty claims if necessary.