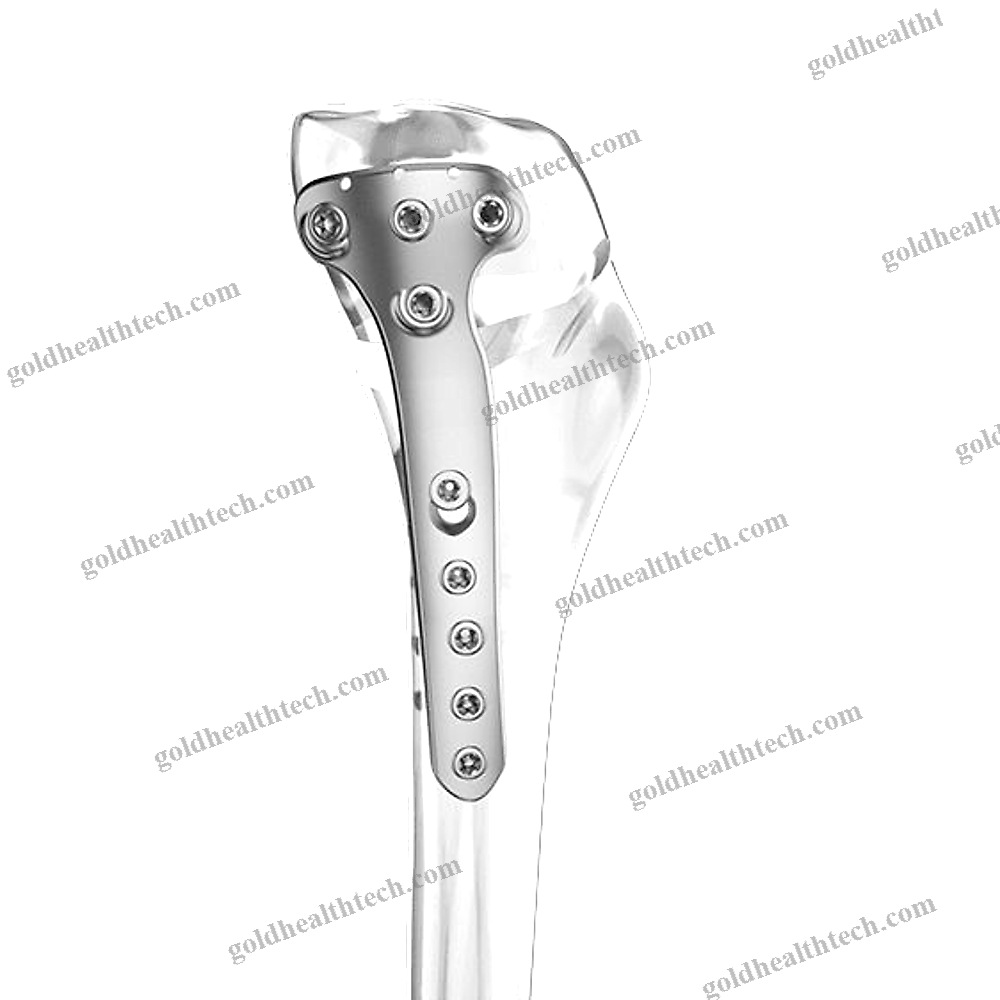
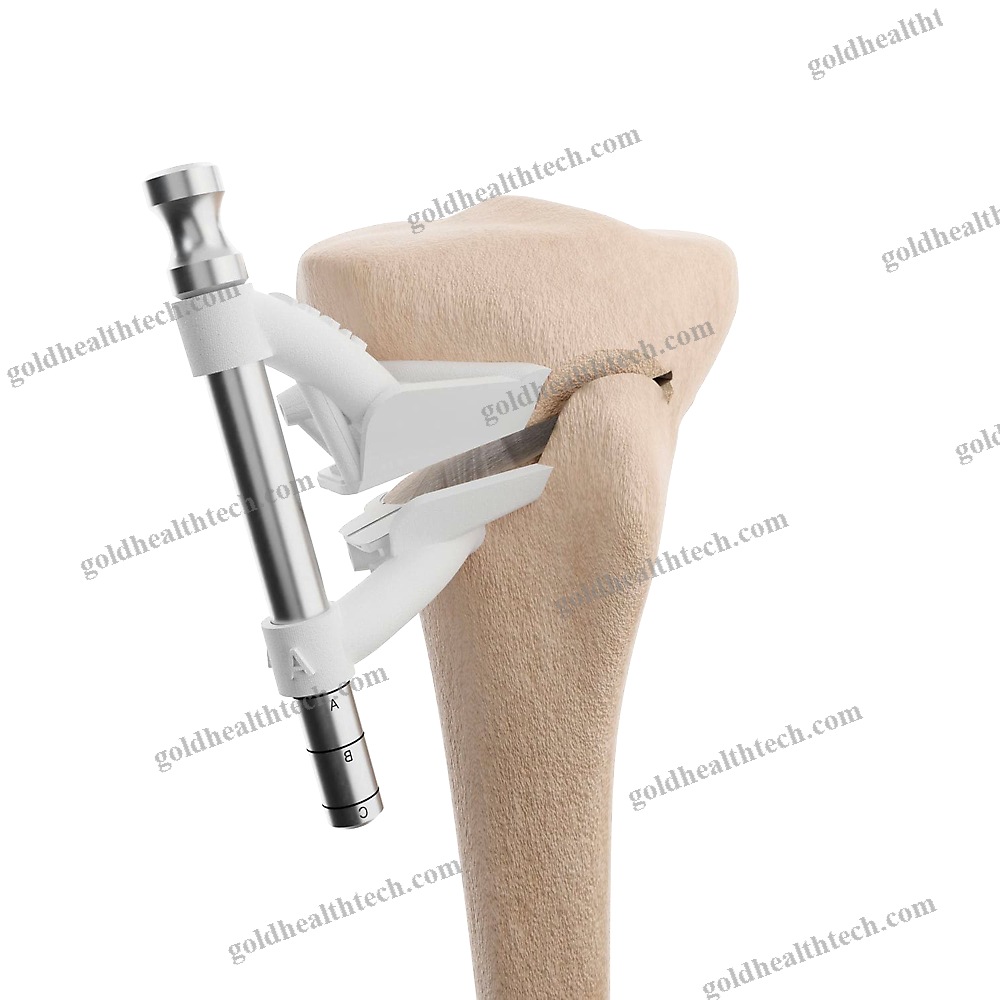
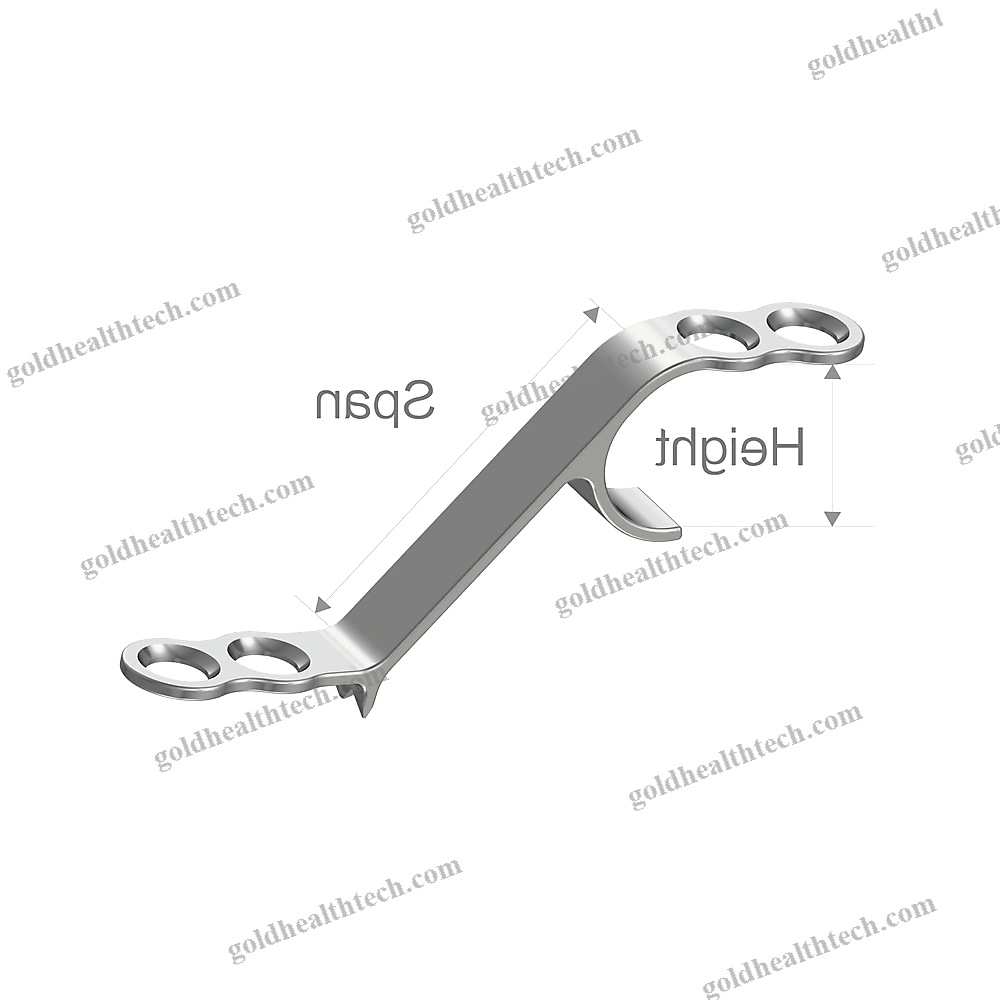
Cylindrical Dental Implant
| Material | Titanium |
| Shape | Cylindrical |
Description
Well-suited for- Limited horizontal bone availability
- Medium and high bone density- crestal sinus liftFeatures: – constructed from medical-grade titanium 5
– flared coronal portion- cylindrical geometry- atraumatic thread design (Standard ISO 5835)- hemispherical apex with two lobes- Three implant diameters (3.3, 4.1, and 4.8 mm)- Four lengths (8, 10, 12, and 14 mm)Package that is sterileOne implant is mounted on a carrier.- One biopolymer cover cap
Shipping & Delivery
Common Shipping and Delivery Considerations
- Packaging:
Medical devices need secure packaging to avoid damage during transit, including shock-absorbing materials or custom foam inserts. - Compliance with Regulations:
Ensure compliance with local and international standards (e.g., FDA, CE) and shipping regulations like Good Distribution Practice (GDP). - Temperature Control:
Some devices require temperature-controlled shipping (e.g., refrigerated trucks or special packaging) to maintain product integrity. - Tracking and Documentation:
Shipments should be tracked with necessary documents like invoices, certificates, and import/export paperwork. - Lead Time:
Plan ahead for lead times, which can vary depending on the equipment size and delivery location. - Insurance: Due to the high value of medical equipment, consider insurance to protect against loss or damage during transit.
- Customs and Import Regulations: Account for customs procedures and tariffs when shipping internationally, ensuring proper documentation.
- Special Handling:
Fragile or hazardous medical devices may require special handling, with clear labeling to ensure proper care. - Delivery Method:
Choose the appropriate shipping method (air, sea, or ground) depending on the equipment’s size, value, and urgency. - Returns and Reverse Logistics:
Have a clear process in place for returns, repairs, and warranty claims if necessary.